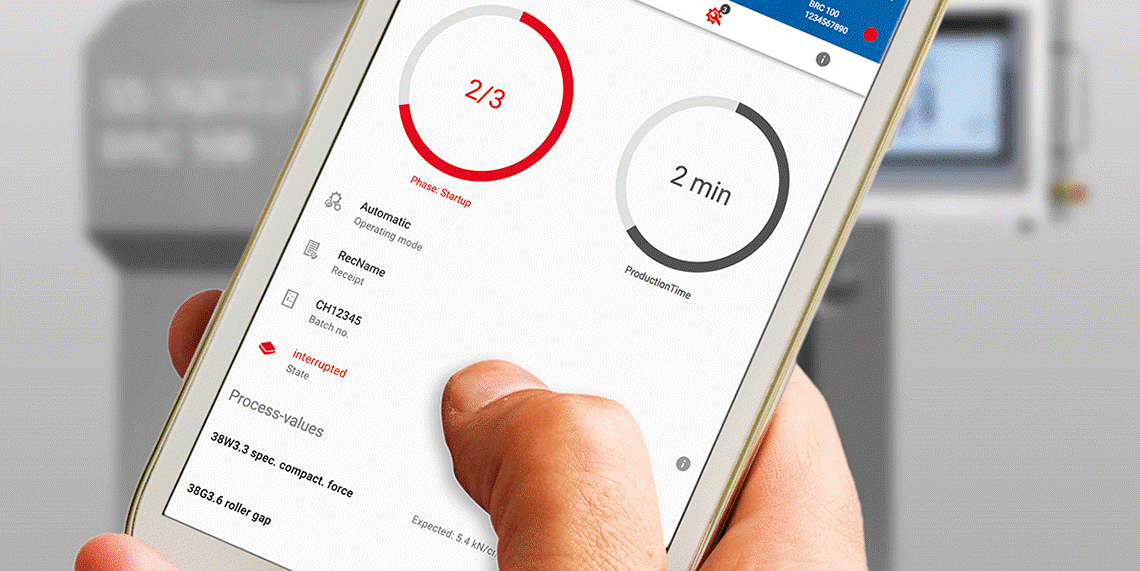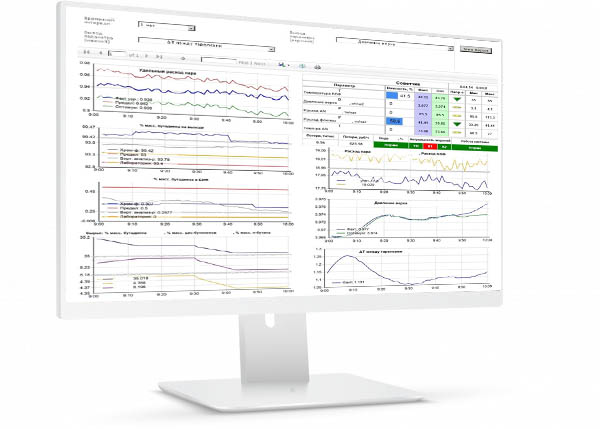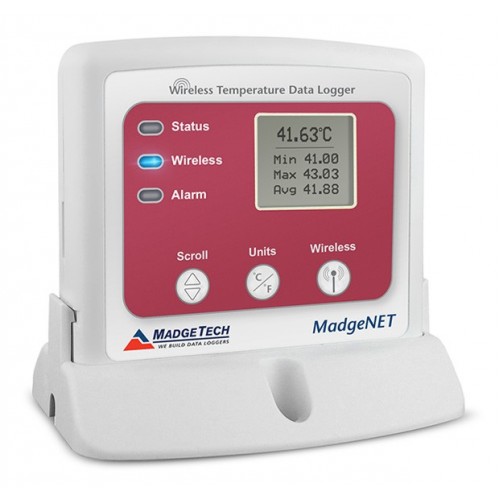
Maintenance of process monitoring systems and drug quality will allow in certain degree to abandon routine revalidation process and routine requalification equipment.
The section presents systems for monitoring the processes and quality of medicines in accordance with GMP.